The Middle East and Africa (MEA) region is emerging as a strategic hub for clinical research activities, driven by improving healthcare infrastructure, favorable regulatory environments, and increasing investments by global pharmaceutical companies. The clinical trial supplies market in MEA plays a pivotal role in enabling the successful execution of clinical studies, ensuring the right drugs, equipment, and logistics are delivered accurately and on time to trial sites.
Full Details Report: https://www.databridgemarketresearch.com/reports/middle-east-and-africa-clinical-trial-supplies-market
Clinical trial supplies encompass a wide range of products and services necessary to conduct clinical trials. This includes investigational medicinal products, comparator drugs, placebo, clinical trial kits, lab supplies, and ancillary materials. The market also comprises services such as packaging, labeling, distribution, logistics, cold chain management, and inventory tracking.
In the MEA region, rising disease burden, increased government initiatives, and the presence of untapped patient populations are encouraging clinical trial sponsors to consider local trial execution. As a result, the demand for efficient and compliant supply chain management solutions has grown substantially. The cost-effectiveness of conducting trials in this region compared to North America and Europe adds to the appeal for pharmaceutical and biotechnology companies.
A significant trend influencing the MEA clinical trial supplies market is the shift toward decentralized and hybrid clinical trials. These models, which incorporate remote monitoring and direct-to-patient delivery, require advanced logistics and supply chain capabilities. The need for temperature-controlled packaging and real-time tracking of shipments is escalating, especially for biologics and gene therapies which are highly sensitive to temperature changes.
Latest Trending Reports:
- Global Hotel Armchair Market
- Global Application Processor Market
- Global Gastric Buttons Market
- Global Medical Thermometers Market
- Global Aluminium Collapsible Tubes Market
- Global Lane Keep Assist System Market
- Global Automotive Refinish Coatings Market
Digitalization and the use of integrated software for inventory management and tracking are also shaping the future of clinical trial supply chains in the region. Automation in packaging, real-time visibility through tracking systems, and advanced forecasting tools are being increasingly adopted. These technologies ensure transparency, compliance with local and international regulations, and overall trial efficiency.
Moreover, regulatory harmonization across various MEA countries is fostering the ease of conducting multinational clinical trials. Countries like the United Arab Emirates, Saudi Arabia, South Africa, and Egypt are streamlining their clinical trial approval processes to attract global pharmaceutical investments. This is leading to the expansion of local logistics and supply chain players who specialize in clinical trial support.
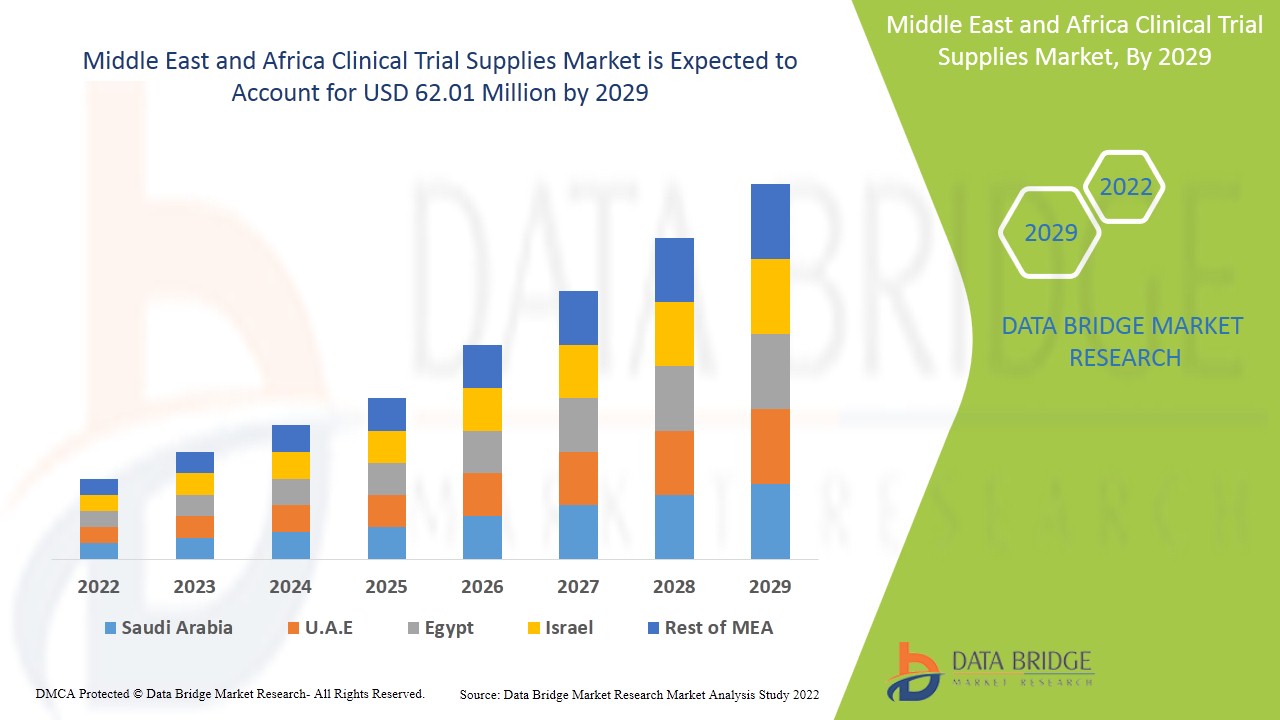
According to recent market analysis, the Middle East and Africa clinical trial supplies market is experiencing a steady growth trajectory. It is projected to grow at a compound annual growth rate (CAGR) of over 6% in the coming years. While this region represents a smaller market compared to North America or Europe, it is among the fastest-growing due to increasing trial activities and government support.
In terms of market size, the MEA clinical trial supplies market is valued in the range of USD 200 to 250 million as of the latest estimates, with significant contributions coming from South Africa, Saudi Arabia, and the UAE. These countries host several trial sites and are witnessing strong collaborations between public and private sector entities. Egypt is also emerging as a key destination, driven by its large patient pool and lower clinical trial costs.
The market share is segmented by product type, service type, phase, therapeutic area, and end-user. Among products, investigational medicinal products (IMPs) dominate the segment, while packaging and labeling services are witnessing strong demand. Oncology, cardiovascular diseases, and infectious diseases are the leading therapeutic areas for clinical trials conducted in the region. Pharmaceutical companies and contract research organizations (CROs) are the major end-users contributing to supply demand.
Growth in the market is also supported by the expansion of global logistics companies into the region. Multinational players are partnering with local distribution agencies to strengthen their capabilities in delivering temperature-sensitive and time-critical clinical trial materials. This collaboration helps in overcoming region-specific challenges such as infrastructure gaps, customs delays, and geopolitical instability.
Demand for clinical trial supplies in the MEA region is being further amplified by the growing interest in rare disease and pediatric trials. These trials require specialized equipment, strict compliance protocols, and patient-centric supply models, adding layers of complexity and increasing the need for expert supply chain services. In response, companies are investing in workforce training, digital tools, and cold chain infrastructure to meet these evolving needs.
Furthermore, the increasing penetration of biotechnology firms in the region is acting as a catalyst for demand. These firms are actively conducting early-phase trials for biosimilars and novel therapeutics. As a result, there is a heightened requirement for customizable and agile supply chain solutions that can accommodate rapid changes in protocol, enrollment rates, and site locations.
With global health challenges such as infectious diseases and emerging pathogens continuing to shape research priorities, MEA is well-positioned to play a larger role in the clinical development pipeline. Governments in the region are recognizing this potential and offering incentives, funding, and partnerships to boost local clinical trial activity and infrastructure.
The future of the clinical trial supplies market in MEA looks promising, supported by a growing ecosystem of sponsors, CROs, and logistics providers. The market is expected to benefit from innovation in packaging technology, demand forecasting, and cloud-based inventory platforms. Investments in local manufacturing of trial materials and warehousing facilities will further contribute to self-sufficiency and reduce reliance on global imports.
About Data Bridge Market Research:
An absolute way to forecast what the future holds is to comprehend the trend today!
Data Bridge Market Research set forth itself as an unconventional and neoteric market research and consulting firm with an unparalleled level of resilience and integrated approaches. We are determined to unearth the best market opportunities and foster efficient information for your business to thrive in the market. Data Bridge endeavors to provide appropriate solutions to the complex business challenges and initiates an effortless decision-making process. Data Bridge is an aftermath of sheer wisdom and experience which was formulated and framed in the year 2015 in Pune.
Contact Us:
Data Bridge Market Research
US: +1 614 591 3140
UK: +44 845 154 9652
APAC : +653 1251 975
Email:- corporatesales@databridgemarketresearch.com